History of Atomic Theory Time Line
By Anthony Tatarka
Democritus
Democritus was a philosopher (460-370BC) that proposed that there might be a ultimate particle. He called this model a atomos. His model was just a particle. No other things like electrons or protons.
Aristotle
Aristotle was a philosipher who came up with the theory that was regardless of the amount of times you cut matter in half you can always get a smaller piece of matter. This theory held for 2000 years because he was known as a great philosipher.
Dalton
John Dalton (1776-1848) came up with the theory called the Law Of Multiple Porportions. He knew that compounds were made up from atoms of elements so he assumed that there are ratios in nature.
Thomson
J.J Thompson(1856-1940) came up with the plum pudding model. The model is made up of a large positive portion with smaller electrons dispersed to keep a neutral charge.
Rutherford
Ernst Rutherford (1871-1937) proposed a nuclear atom because of the gold foil experiment. He also proposed that all of the mass and the positively charged particles were in the center of the atom while electrons took up most of the empty space.
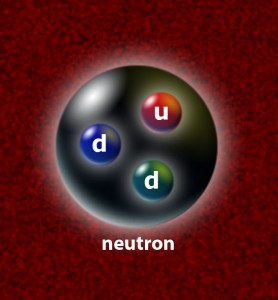
In 1920 he also proposed a third particle, the neutron.
Bohr
Bohr (1885-1962) proposed that the atom was a positively charged nucleus surrounded by electrons. He also found out electrons travel in different pathst around the nucleus and the different number of electrons determine the element..
Modern Atomic Theory Model - James Chadwick
James Chadwick (1891-1974) , a collaborator of Rutherford, discovered the neutron in 1932. This led to the discovery of nueclear fission.