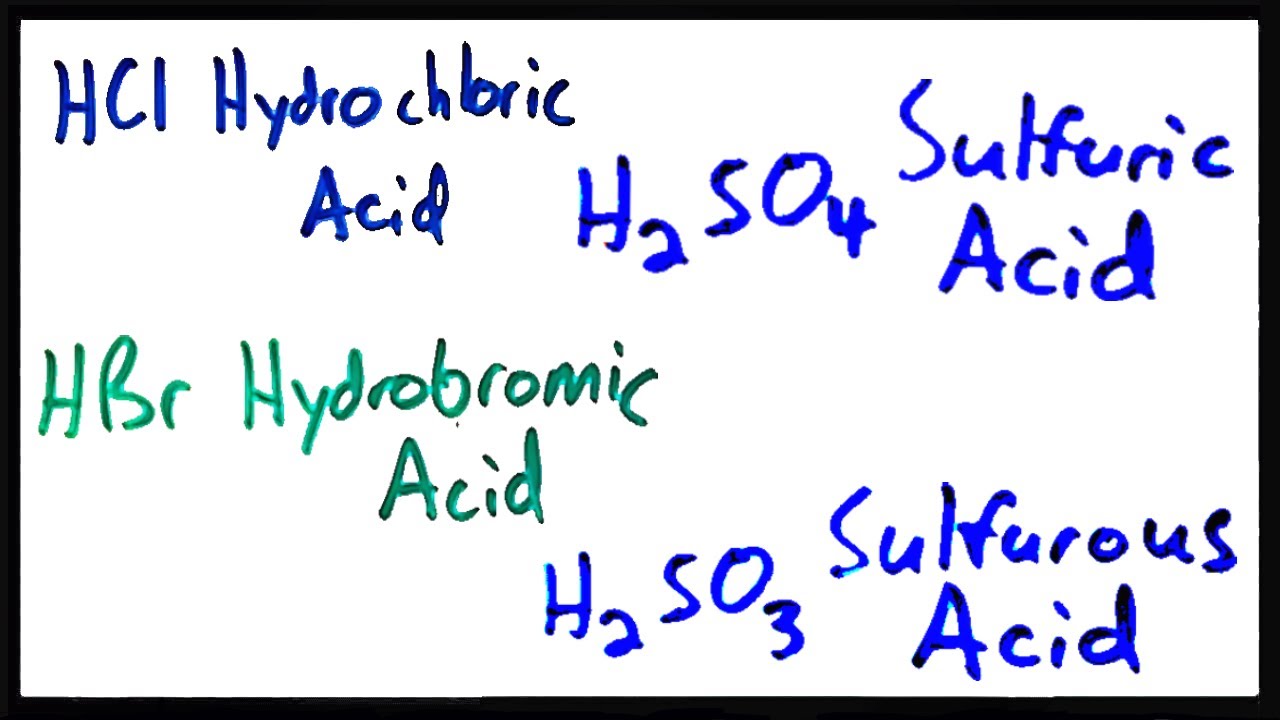
Naming acids
Acids nomenclature
How to recognize an acid
*You can recognize an acid by the fact that it's formula starts with H
- HCl -HNO3 -H2SO4
Some acids formula don't start with H
*H2O is not considered an acid
Recognize polyatomic ions
1)Example ClO
Hypochlorite ( "ite" suffix is changed to "ous" and you ass the word acid)
2)
3)
Binary acids
1) prefix "hydro" is used
2) root of the anion is used
3) suffix "ic" is used
4) the word "acid" is used as the second word in the name
Practice problems
1)*Nitric acid
2)*Chloric acid
3)*Acetic acid
4)*Hydrobromic acid
5)*Sulfurous acid
6)*Chlorous acid
7)*Hydrochloric acid
8)*Phosphoric acid
9)*Nitrous acid
10)*Hydrofluoric acid
11)*Hypochlorous acid
12)*Hydroiodic acid
13)*Phosphorous acid
14)*Carbonic acid
15)*Perchloric acid
16)*Permanganic acid
17)*Sulfuric acid
18)*Hydrocyanic acid
Answers
1)HNO3
2)HClO3
3)HC2H3O2 (or HCH3COO, or HCH3CO2)
4) HBr
5)H2SO3
6)HClO2
7)HCl
8)H3PO4
9)HNO2
10) HF
11) HClO
12) HI
13) H3PO3
14) H2CO3
15) HClO4
16) HMnO4
17) H2SO4
18) HCN